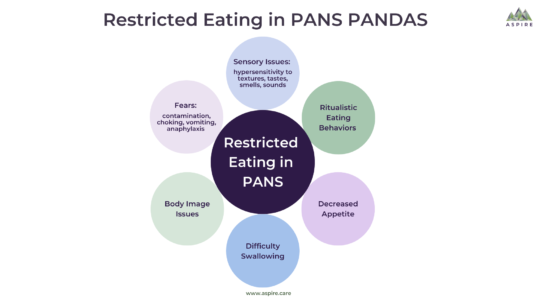
Haylie’s Healing Journey: A Story of Strength and Resilience
The key to treatment was aggressive intervention. But finding the right doctors was a battle. Most practitioners were unaware of PANS or didn’t believe in its severity. We were lucky to get a...